Table of Contents
ToggleIntroduction
As medical devices increasingly become portable, wearable, and implanted, polymer lithium batteries (LiPo batteries) have emerged as the preferred power source due to their lightweight construction, flexible form factors, and high energy density. This evolution enables the development of smaller, smarter, and more user-friendly medical instruments, improving patient care quality and convenience. However, the adoption of custom lithium polymer batteries in medical devices carries crucial safety implications given the sensitive nature of their application and the inherent risks of lithium battery chemistry.
This comprehensive article investigates the question: Is it safe to use custom lithium polymer batteries in medical devices? It explores the safety challenges, regulatory frameworks, key standards, manufacturing processes, and quality control measures essential for ensuring battery safety in medical applications. Insights from industry leaders like Lipobattery SY (lipobatterysy.com) reinforce the practical aspects of design, testing, certification, and ongoing monitoring.

The Importance of Battery Safety in Medical Devices
Medical devices differ fundamentally from consumer electronics in their direct or indirect impact on human health. Any device malfunction or power failure can result in compromised treatment efficacy or even endanger lives. Given this, battery safety in medical devices is paramount to ensure surging demands for portable power do not compromise reliability or patient welfare.
Lithium polymer batteries provide unmatched advantages in energy density and packaging flexibility but involve risks such as:
-
Thermal runaway and combustion due to electrochemical instability or short circuits, which can cause fire or explosions.
-
Risks stemming from overcharge, over-discharge, or electrical faults, potentially damaging the battery or device.
-
Possibility of chemical leakage from electrolyte materials affecting device integrity and user safety.
-
Effects of physical or mechanical abuse, such as crushing or puncturing, which may compromise internal battery structures.
The challenge multiplies with custom battery designs, which may deviate from tested commercial standards to fit specialized form factors needed in medical wearables, implantables, and monitoring systems.
Why Custom Lithium Polymer Batteries Are Chosen for Medical Devices
Despite the challenges, many medical device manufacturers prefer custom lithium polymer batteries due to their unique benefits:
-
Precision form factor customization enabling ultra-thin, contoured, or irregular battery shapes matching device enclosures closely.
-
Tailored capacity and voltage configurations optimizing device runtime without excess weight or volume.
-
Integration with advanced Battery Management Systems (BMS) and protection circuits calibrated for the specific device use case.
-
Improvements in mechanical robustness and thermal management adapted to device operating environments.
-
Ability to implement biocompatible materials and insulation essential for devices in contact with or implanted in the human body.
These enhancements promote device miniaturization, improve patient comfort, and support longer battery lifecycles. However, they demand meticulous design, testing, and certification to guarantee safety.
Regulatory Landscape and Safety Standards Impacting Custom LiPo Batteries in Medical Devices
Regulatory bodies and international standards define strict compliance requirements for batteries embedded in medical devices. These ensure risks are minimized and products deliver consistent safe performance. The primary standards and certifications to consider are:
IEC 62133: Safety Requirements for Rechargeable Cells and Batteries
IEC 62133 is a foundational standard detailing:
-
Tests for overcharge, over-discharge, external short circuit, and forced internal failure conditions.
-
Mechanical abuse testing such as crushing, impact, vibration, and shock.
-
Thermal stability under high and low temperature extremes.
-
Design requirements for protection circuits, including cutoffs and monitoring.
-
Specifications on marking, documentation, and quality traceability.
This standard applies directly to polymer lithium batteries in medical devices, ensuring they meet abuse resistance and electrical safety thresholds vital for patient protection.

UL Standards: UL 2054 and UL 1642
-
UL 2054 applies to battery packs including polymer lithium batteries used in household and commercial equipment; it emphasizes electrical and fire safety through extensive tests.
-
UL 1642 applies to lithium batteries themselves, testing for resistance to electrical, fire, and mechanical abuse.
These certifications are frequently requested by the US FDA as evidence of safety and are widely accepted internationally.
UN 38.3: Lithium Battery Transportation Safety Testing
Applicable to all lithium batteries in transit, UN 38.3 certifies batteries through:
-
Altitude simulation.
-
Thermal cycling.
-
Mechanical shock and vibration.
-
External short circuit.
-
Impact and overcharge.
Ensuring shipping safety is crucial because destruction, decomposition, or fire during transport carries risks for personnel and infrastructure.
IEC 60601 Series: Medical Electrical Equipment Safety
IEC 60601 defines electrical safety for medical equipment, with relevant parts covering:
-
Leakage current limits to protect patients, especially in devices with direct patient contact.
-
Isolation and insulation requirements protecting against electrical hazards.
-
Requirements regarding electromagnetic compatibility (EMC), crucial to prevent interference affecting device reliability.
Custom lithium polymer battery packs must be designed and integrated considering these system-level requirements to preserve overall device safety.
Other Relevant Standards and Approvals
-
ISO 10993: Biocompatibility testing of materials used in medical devices.
-
ISO 14971: Risk management framework for medical devices.
-
RoHS and REACH compliance to limit hazardous substances.
-
Regional certification like CFDA (China), MDR (Europe), or Health Canada approvals.
Risks and Challenges in Custom LiPo Battery Development for Medical Devices
Designing custom lithium polymer batteries presents several technical and regulatory challenges:
Manufacturing Precision and Consistency
Deviations from standard cell shapes require specialized tooling and assembly processes, potentially impacting uniformity in:
-
Cell thickness and layer dimensions.
-
Electrode alignment.
-
Electrolyte distribution and sealing.
Inconsistent manufacturing can increase risks of internal shorts or gas generation.
Designing for Failure Modes and Protections
Custom batteries must be validated for:
-
Overcharge and overdischarge scenarios, which can cause irreversible damage or safety incidents.
-
Sensitivity to mechanical shock and vibration inherent to patient movement or device usage.
-
Thermal management suitable to ambient and operating environments, avoiding hotspots or thermal runaway.
Protection circuits tailored to device power profiles and operational software safeguards must be integrated and validated.
Biocompatibility and Packaging
Wearable or implantable devices must ensure that the battery materials in contact with skin or tissue are:
-
Chemically non-reactive.
-
Hypoallergenic.
-
Durable against sweat, moisture, and body fluids.
Secondary packaging layers and seals must prevent electrolyte leakage.
Regulatory and Certification Burden
Each custom battery design iteration potentially requires full retesting and recertification, increasing cost and time to market. Variations in battery sizes, chemistries, or protection methodologies complicate cross-regional approvals.

Long-Term Stability and Reliability Testing
Medical devices often have stringent lifecycle expectations. Custom LiPo batteries must undergo accelerated aging, cycle testing, and shelf-life assessments to guarantee minimal capacity loss and stable voltage profile over device lifespan.
Table: Key Safety and Regulatory Aspects for Custom Polymer Lithium Batteries in Medical Devices
| Aspect | Description | Medical Impact | Required Compliance / Tests |
|---|---|---|---|
| Electrical Safety | Resilience to overcharge, overdischarge, short circuit | Prevent battery fires and device damage | IEC 62133, UL 2054/1642 (Cell-level tests) |
| Mechanical Abuse Resistance | Ability to withstand shocks, vibration, crushing | Avoid internal damage, short circuits | IEC 62133, UL mechanical testing procedures |
| Thermal Stability | Operability under temperature extremes | Prevent thermal runaway and safe usage | IEC 62133, UN 38.3 thermal tests |
| Biocompatibility | Compatibility of battery materials with body/skin contact | Avoid skin irritation or toxicity | ISO 10993 standard testing |
| Transportation Safety | Meets shipping safety for international logistics | Enables global distribution | UN 38.3 certification |
| Battery Management System (BMS) | Hardware/software protection to monitor battery state | Enhances safety, longevity | Functional verification, IEC 62133 |
| Electrical Isolation / Leakage | Limits leakage current for patient safety | Protects patient from electrical shock | IEC 60601 device-level requirements |
| Quality Management | Structured manufacturing, traceability, and documentation | Ensures consistent product safety and recall ability | ISO 9001 certified manufacturing practices |
| Environmental Compliance | Absence of hazardous substances | Safeguards environment and patient health | RoHS, REACH compliance |
| EMC Compliance | Regulation of electromagnetic emissions | Ensures device reliability | IEC 60601-1-2, FDA guidelines |
Best Practices for Safe Custom Lithium Polymer Battery Use in Medical Devices
Start with Certified Base Cells
Use commercially certified cells already compliant with IEC 62133 and UL to mitigate base chemistry risks.
Partner with Experienced Battery Manufacturers
Choose suppliers like Lipobattery SY (lipobatterysy.com) who specialize in medical-grade custom polymer lithium batteries with documented quality systems and certification support.
Rigorous Design Verification and Validation
Conduct extensive lab testing for electrical, mechanical, environmental, and chemical stability per regulatory standards. Include worst-case abuse scenario evaluations.
Integrate High-Performance BMS
Implement battery management systems with:
-
Voltage and current monitoring.
-
Cell balancing.
-
Thermal detection and cutoff.
-
Communication with host device for real-time safety management.
Prioritize Biocompatible Materials and Packaging
Select materials passing ISO 10993 or equivalent and validate encapsulation against body fluid ingress and mechanical strain.
Continuous Quality Assurance and Traceability
Maintain batch-level traceability and conduct factory audits aligning with ISO 9001 quality management systems to ensure production consistency.
Plan for Lifecycle and Aging Studies
Perform long-term cycling and shelf-life testing matching expected device usage profiles to forecast capacity retention and safety over time.
Clear and Complete Regulatory Documentation
Support device regulatory filings with comprehensive battery technical files, including certificates, test reports, risk analyses, and manufacturing controls.
Industry Examples of Safe Custom Polymer Lithium Battery Use
Wearable Health Monitors
Manufacturers develop thin, contoured polymer lithium batteries with protections guaranteeing stable operation for multiple days, meeting IEC 62133 and UL standards. These batteries enable ergonomically designed wearable devices with safe skin contact, low leakage currents, and long cycle lives.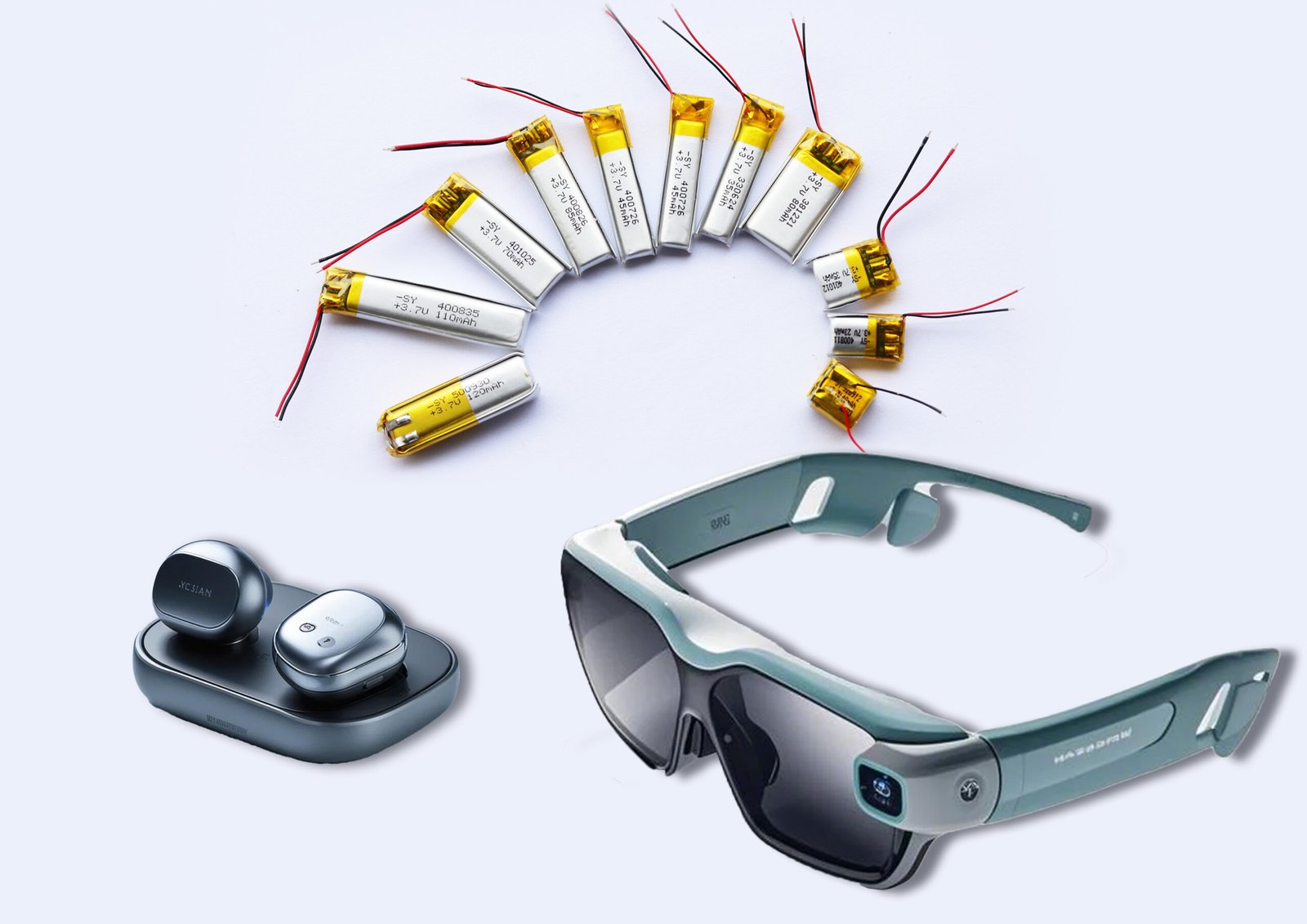

Portable Defibrillators
Custom LiPo batteries optimized for high discharge currents provide reliable energy bursts during resuscitation, validated through rigorous thermal and mechanical abuse testing. Protection circuits ensure operational and post-incident battery safety.
Implantable Neurostimulators
Custom-shaped batteries undergo exhaustive biocompatibility, hermetic sealing, and long-term aging studies, designed to operate safely within body fluids for years without replacement. Multi-level system safety architecture mitigates failure risks.
Conclusion
Using custom lithium polymer batteries in medical devices can be safe when stringent design, manufacturing, and certification standards are strictly followed. The unique advantages of LiPo batteries in form factor and energy density must be balanced against the critical safety requirements specific to medical applications.
A holistic approach encompassing:
-
Use of certified components.
-
Robust safety design with integrated battery management.
-
Thorough compliance with regulatory standards such as IEC 62133, UL, UN38.3, and IEC 60601.
-
Extensive safety and durability testing.
-
Compliance with biocompatibility and environmental standards.
-
Reliable supply chain with traceability.
is essential to ensure patient protection and device reliability.
Lipobattery SY (lipobatterysy.com) exemplifies industry best practices, offering custom polymer lithium battery solutions that meet medical requirements globally with certified safety, quality, and technical support.
For manufacturers and designers seeking to leverage the benefits of custom lithium polymer batteries confidently in medical devices, partnering with experienced suppliers and steadfastly adhering to safety frameworks is the foundation of success.

